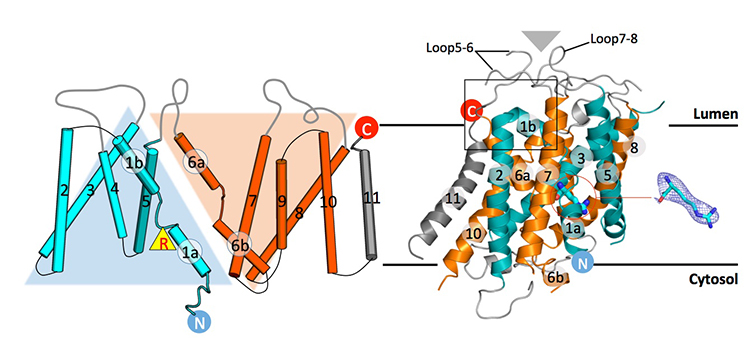
Recent advances in intracellular amino acid transport and mechanistic target of rapamycin complex 1 (mTORC1) signaling shed light on the solute carrier 38 family A member 9 (SLC38A9), a lysosomal transporter responsible for binding and translocation of several essential amino acids. Here we present the first crystal structure of SLC38A9 from Danio rerio in complex with arginine. As captured in the cytosol-open state, the bound arginine was locked in a transitional state stabilized by the transmembrane helix 1 (TM1) of drSLC38A9 which was anchored at the grove between TM5 and TM7. These anchoring interactions were mediated by the highly conserved motif WNTMM on TM1 and mutations in this motif abolished arginine transport by drSLC38A9. The underlying mechanism of substrate binding is critical for sensitizing mTORC1 signaling pathway to amino acids and for maintaining lysosomal amino acid homeostasis. This study offers a first glimpse into a prototypical model for SLC38 transporter.
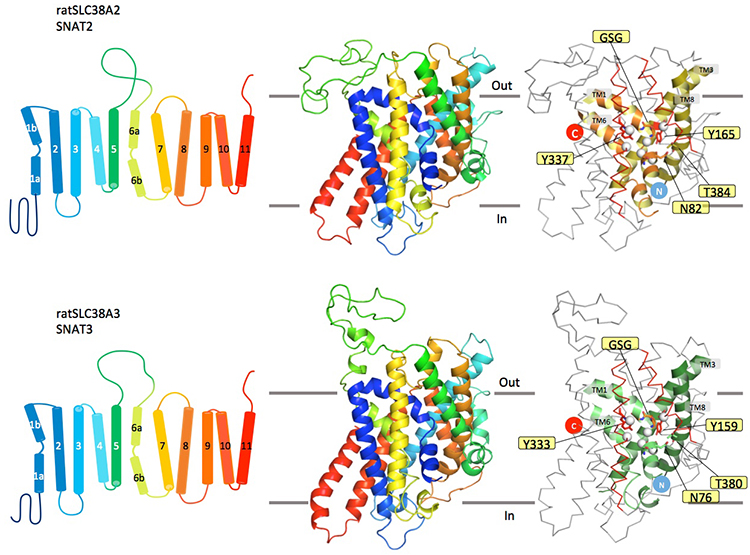
Using this initial structure, we could compute models for representative SNAT proteins.
Relevant papers
A conformational change in the N Terminus of SLC38A9 signals mTORC1 activation
In: Structure, vol. 29, no. 5, pp. 426–432.e8, 2021.
Crystal structure of arginine-bound lysosomal transporter SLC38A9 in the cytosol-open state
In: Nat. Struct. Mol. Biol., vol. 25, no. 6, pp. 522–527, 2018.