Membrane Protein Complexes
Our structure of the AQP0/CaM complex is the first for any full-length membrane channel in complex with this ubiquitous secondary messenger. Current efforts in the laboratory are to understand how Ca²⁺/CaM binds to and modulates the activity of other channels such as ion channels.
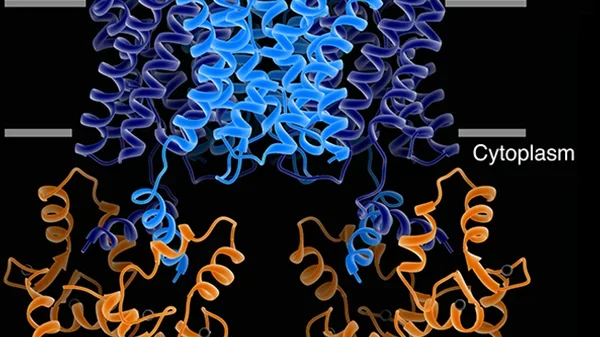
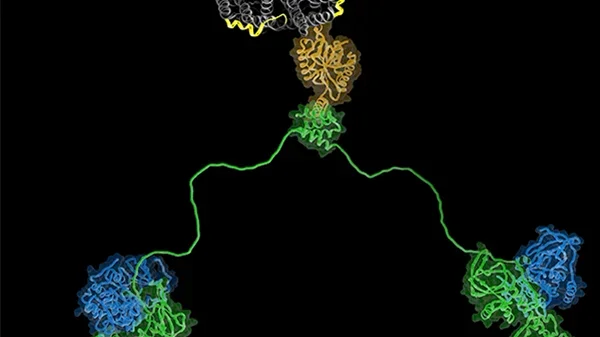
We are also trying to understand more about the AQP-AKAP system, in particular we are trying to assemble the AQP2-AKAP18-PKA complex and AQP0-AKAP2-PKA complex for structural studies. Intrinsically disordered regions of proteins are widespread in nature yet the mechanistic roles they play in biology are underappreciated. Such disordered segments can act simply to link functionally coupled structural domains or they can orchestrate enzymatic reactions through a variety of allosteric mechanisms. The regulatory subunits of protein kinase A provide an example of this important phenomenon where functionally defined and structurally conserved domains are connected by intrinsically disordered regions of defined length with limited sequence identity. Our studies show that this seemingly paradoxical amalgam of order and disorder permits fine-tuning of local protein phosphorylation events. The anchoring of PKA by AKAP affords the kinase a sphere of action in which multiple targets can get phosphorylated fast in a cAMP independent way.
Relevant Papers
Smith, Donelson F; Reichow, Steve L; Esseltine, Jessica L; Shi, Dan; Langeberg, Lorene K; Scott, John D; Gonen, Tamir
Intrinsic disorder within an AKAP-protein kinase A complex guides local substrate phosphorylation
In: Elife, vol. 2, pp. e01319, 2013.
Reichow, Steve L; Clemens, Daniel M; Freites, Alfredo J; Nemeth-Cahalan, Karin L; Heyden, Matthias; Tobias, Douglas J; Hall, James E; Gonen, Tamir
Allosteric mechanism of water-channel gating by Ca²⁺-calmodulin
In: Nat. Struct. Mol. Biol., vol. 20, no. 9, pp. 1085–1092, 2013.
Gold, Matthew G; Reichow, Steve L; O'Neill, Susan E; Weisbrod, Chad R; Langeberg, Lorene K; Bruce, James E; Gonen, Tamir; Scott, John D
AKAP2 anchors PKA with aquaporin-0 to support ocular lens transparency
In: EMBO Mol Med, vol. 4, no. 1, pp. 15–26, 2011.
Reichow, Steve L; Gonen, Tamir
Noncanonical Binding of Calmodulin to Aquaporin-0: Implications for Channel Regulation
In: Structure, vol. 16, no. 9, pp. 1389–1398, 2008.
Gonen, Tamir; Cheng, Yifan; Sliz, Piotr; Hiroaki, Yoko; Fujiyoshi, Yoshinori; Harrison, Stephen C; Walz, Thomas
Lipid-protein interactions in double-layered two-dimensional AQP0 crystals
In: Nature, vol. 438, no. 7068, pp. 633–638, 2005.
Gonen, Tamir; Cheng, Yifan; Kistler, Joerg; Walz, Thomas
Aquaporin-0 Membrane Junctions Form Upon Proteolytic Cleavage
In: J. Mol. Biol., vol. 342, no. 4, pp. 1337–1345, 2004.
Gonen, Tamir; Sliz, Piotr; Kistler, Joerg; Cheng, Yifan; Walz, Thomas
Aquaporin-0 membrane junctions reveal the structure of a closed water pore
In: Nature, vol. 429, no. 6988, pp. 193–197, 2004.
Relevant Reviews
Gold, Matthew G; Gonen, Tamir; Scott, John D
Local cAMP signaling in disease at a glance
In: J. Cell. Sci., vol. 126, no. Pt 20, pp. 4537–4543, 2013.
Reichow, Steve L; Gonen, Tamir
Lipid-protein interactions probed by electron crystallography
In: Curr. Opin. Struct. Biol., vol. 19, no. 5, pp. 560–565, 2009.
Andrews, Simeon; Reichow, Steve L.; Gonen, Tamir
Electron Crystallography of Aquaporins
In: IUBMB Life, vol. 60, no. 7, pp. 430–436, 2008.
Engel, Andreas; Fujiyoshi, Yoshinori; Gonen, Tamir; Walz, Thomas
Junction-forming aquaporins
In: Curr. Opin. Struct. Biol., vol. 18, no. 2, pp. 229–235, 2008.
Gonen, Tamir; Walz, Thomas
The structure of aquaporins
In: Q. Rev. Biophys., vol. 39, no. 4, pp. 361–396, 2006.