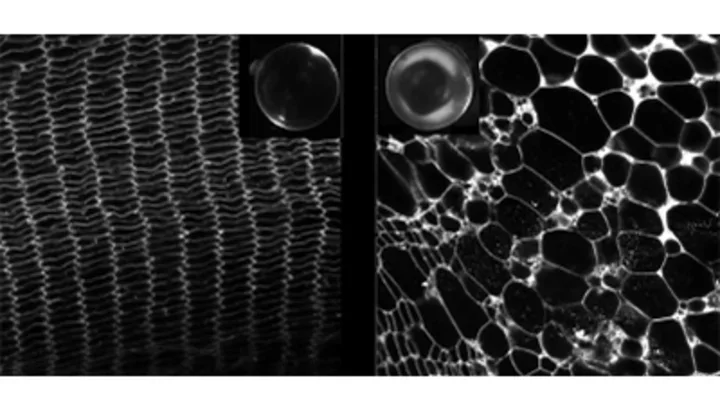
Water Permeation
Water channels, or aquaporins, form specialized channels in membranes for water permeation. These are extremely efficient channels that allow millions of water molecules to permeate the pore per second. Because they are channels, the cell can regulate their activity dynamically to help maintain homeostasis. In the case of the eye lens water channel aquaporin-0 (AQP0), it can be regulated by at least four known mechanisms that we studied over the last decade. The first is irreversible and involves the cleavage of the C-terminal domain of AQP0. The cleavage results in complete pore closure and AQP0 ceases to act as a water channel. Instead, it becomes an adhesive protein mediating cell-to-cell adhesive junctions.
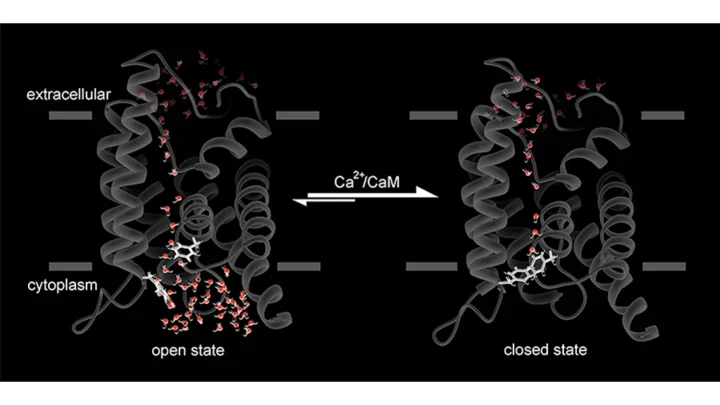
Full-length AQP0 is dynamically modulated by three mechanisms: pH, calcium/calmodulin (Ca²⁺/CaM) and protein phosphorylation. We recently showed that the binding of Ca²⁺/CaM to AQP0 results in partial pore closure. The net effect is that the permeability through AQP0 halves in the presence of Ca²⁺/CaM. Conversely, we showed that phosphorylation of AQP0 by anchored PKA (AKAP2/PKA complex) abolished CaM binding, keeping AQP0 in the open conformation and functioning at maximal activity.
Our studies of channel phosphorylation led us to discover a new protein in the eye lens called AKAP2. Our biochemical and structural studies indicate that AKAPs anchor PKA onto substrate and provide the kinase a sphere of action in which the kinase could phosphorylate substrates in a cAMP independent way. This is fundamentally an exciting observation because it helps explain how fast phosphorylation can occur, as seen for example in heart cells. Moreover, we showed that inhibition of phosphorylation of AQP0 in the lens results in cataract formation. Essentially, we recapitulated the lens disease ex vivo by inhibiting protein phosphorylation.