Vibrio cholera toxin secretion channel
In collaboration with Wim Hol (UW) we studied the structure of the vibrio cholera toxin secretion channel.
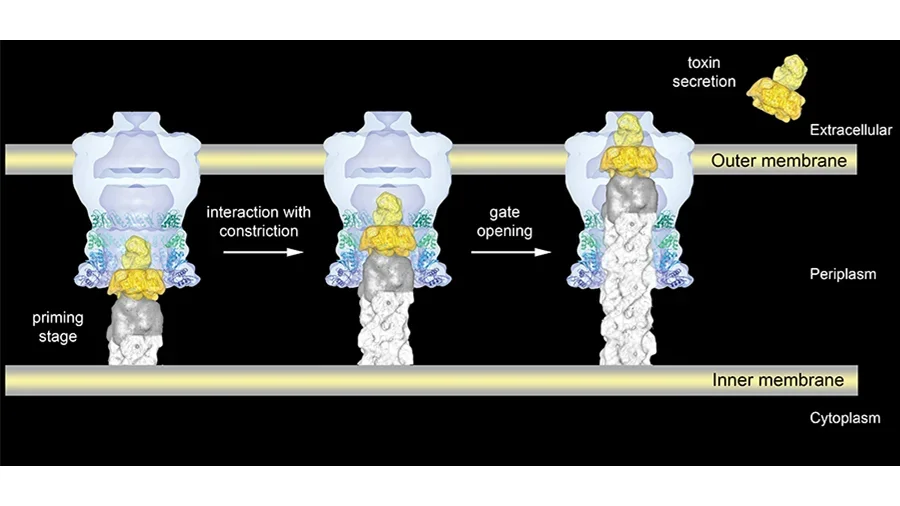
The type II secretion system (T2SS) is a macromolecular complex spanning the bacterial inner and outer membranes of Gram-negative bacteria, including many pathogenic bacteria such as Vibrio cholerae and enterotoxigenic Escherichia coli. The T2SS secretes folded proteins including cholera toxin and heat-labile enterotoxin. The major outer membrane T2SS protein is the “secretin” GspD. Electron cryomicroscopy (cryo-EM) reconstruction of the V. cholerae secretin at 19 Å resolution reveals a dodecameric structure reminiscent of a barrel with a large channel at its center that appears to be in a closed state. On the periplasmic side of the channel vestibule contains both a constriction and a gate. On the extracellular side a large chamber is enclosed by a cap structure. By combining our results with structural data on a large exoprotein and the dimensions of the tip of the pseudopilus of the T2SS, we provide a structural basis for a possible secretion mechanism of exoproteins by the T2SS in which the constriction site plays a critical role.
Relevant papers
Reichow, Steve L; Korotkov, Konstantin V; Gonen, Melissa; Sun, Ji; Delarosa, Jaclyn R; Hol, Wim G J; Gonen, Tamir
The binding of cholera toxin to the periplasmic vestibule of the type II secretion channel
In: Channels (Austin), vol. 5, no. 3, pp. 215–218, 2011.
Reichow, Steve L; Korotkov, Konstantin V; Hol, Wim G J; Gonen, Tamir
Structure of the cholera toxin secretion channel in its closed state
In: Nat. Struct. Mol. Biol., vol. 17, no. 10, pp. 1226–1232, 2010.
Relevant reviews
Korotkov, Konstantin V; Gonen, Tamir; Hol, Wim G J
Secretins: dynamic channels for protein transport across membranes
In: Trends Biochem. Sci., vol. 36, no. 8, pp. 433–443, 2011.