Self‐Assembling Proteins
Computational design of genetically encoded self‐assembling proteins
In collaboration with David Baker (HHMI, UW) we are designing genetically encoded self assembling proteins for cellular microcircuitry.
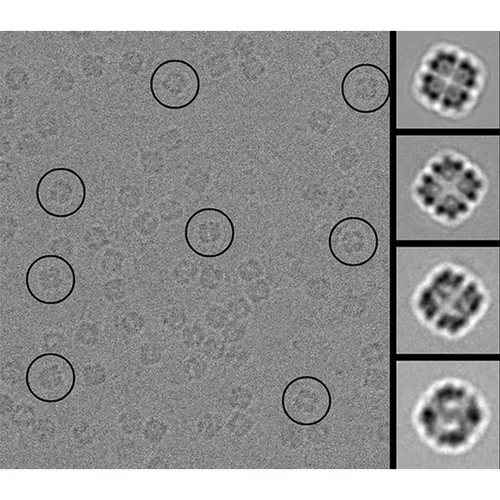
We describe a general computational method for designing proteins that self-assemble to a desired symmetric architecture. Protein building blocks are docked together symmetrically to identify complementary packing arrangements, and low-energy protein-protein interfaces are then designed between the building blocks in order to drive self-assembly. Here we use trimeric protein building blocks to design a 24-subunit, 13 nm diameter complex with octahedral symmetry and two related variants of a 12-subunit, 11 nm diameter complex with tetrahedral symmetry. The designed proteins assembled to the desired oligomeric states in solution, and crystal structures of the complexes revealed that the resulting materials closely match the design models. The method can be used to design a wide variety of self-assembling protein nanomaterials.
Relevant papers
Bale, Jacob B; Park, Rachel U; Liu, Yuxi; Gonen, Shane; Gonen, Tamir; Cascio, Duilio; King, Neil P; Yeates, Todd O; Baker, David
Structure of a designed tetrahedral protein assembly variant engineered to have improved soluble expression
In: Protein Sci., vol. 24, no. 10, pp. 1695–1701, 2015.
Gonen, Shane; DiMaio, Frank; Gonen, Tamir; Baker, David
Design of ordered two-dimensional arrays mediated by noncovalent protein-protein interfaces
In: Science, vol. 348, no. 6241, pp. 1365–1368, 2015.
King, Neil P; Bale, Jacob B; Sheffler, William; McNamara, Dan E; Gonen, Shane; Gonen, Tamir; Yeates, Todd O; Baker, David
Accurate design of co-assembling multi-component protein nanomaterials
In: Nature, vol. 510, no. 7503, pp. 103–108, 2014.
King, Neil P; Sheffler, William; Sawaya, Michael R; Vollmar, Breanna S; Sumida, John P; André, Ingemar; Gonen, Tamir; Yeates, Todd O; Baker, David
Computational Design of Self-Assembling Protein Nanomaterials with Atomic Level Accuracy
In: Science, vol. 336, no. 6085, pp. 1171–1174, 2012.