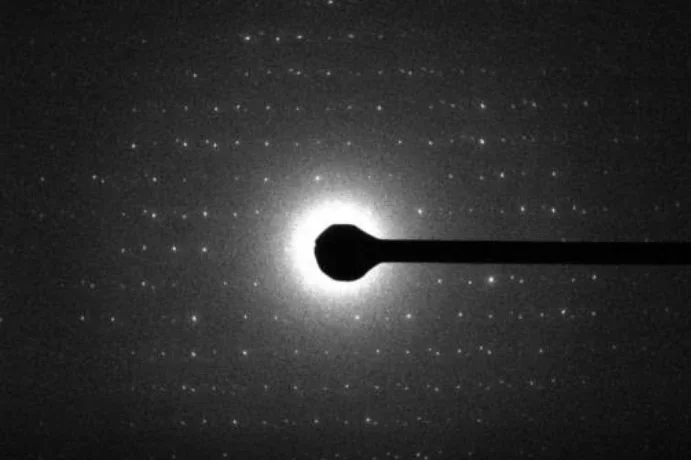
MicroED

Interview with Tamir Gonen about MicroED
We demonstrated that it is feasible to determine high-resolution protein structures by electron crystallography of three-dimensional crystals in an electron cryo-microscope (cryo-EM). Lysozyme microcrystals were frozen on an electron microscopy grid, and electron diffraction data collected to 1.7 Å resolution. We developed a data collection protocol to collect a full-tilt series in electron diffraction to atomic resolution. A single tilt series contains up to 90 individual diffraction patterns collected from a single crystal with tilt angle increment of 0.1°–1° and a total accumulated electron dose less than 10 electrons per Å2. We indexed the data from three crystals and used them for structure determination of lysozyme by molecular replacement followed by crystallographic refinement to 2.9 Å resolution. This proof of principle paves the way for the implementation of a new technique, which we name MicroED, that may have wide applicability in structural biology.
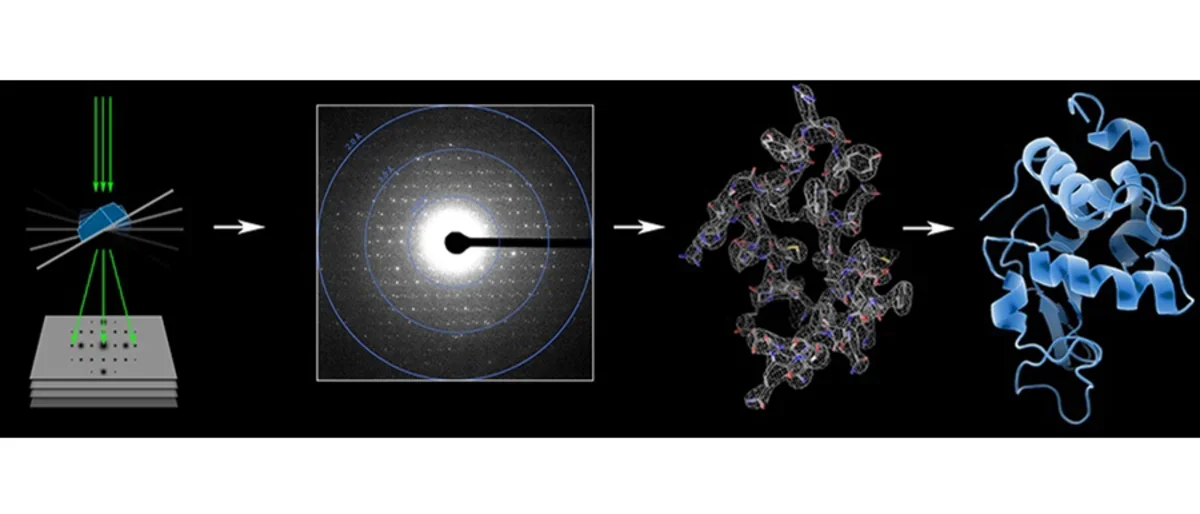
In 2014 we further improved the MicroED method. Firstly, we developed an improved data collection protocol for MicroED called continuous rotation. Microcrystals are continuously rotated during data collection yielding improved data and allowing data processing with the crystallographic software tool MOSFLM, resulting in improved resolution for the model protein lysozyme to 2.5 Å resolution. These improvements pave the way for the broad implementation and application of MicroED in structural biology. Current efforts include new phasing methods, automation, and program development.
Secondly, we used the improved MicroED protocols for data collection and analysis to determine the structure of catalase. Bovine liver catalase crystals that were only ~160 nm thick were used for the structure analysis. A single crystal yielded data to 3.2 Å resolution enabling rapid structure determination.
In 2015 we published the first two previously unknown structures determined by MicroED. The structures of two peptides from the toxic core of α-synuclein of Parkinson’s disease. The structures were determined from vanishingly small crystals, only ~200 nm thick and wide, and yielded 1.4 Å resolution. These structures, which are currently the highest resolution structures determined to date by any cryo-EM method, show new and important structural information that could aid in the development of pharmaceuticals against this devastating neurological disease. The study, which was published by Nature, also show a number of protons for the very first time.
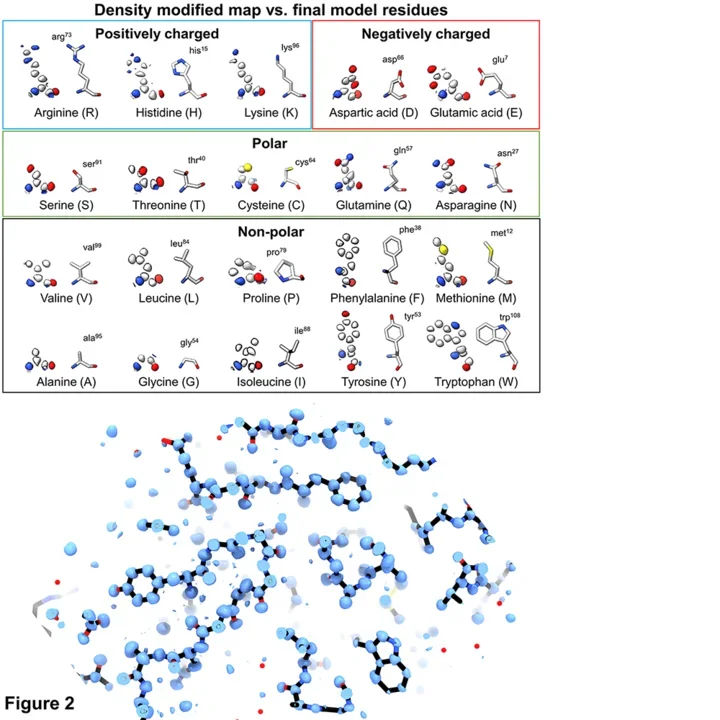
2021: Structures of two globular proteins were determined ab initio using microcrystal electron diffraction (MicroED) data. Microcrystals were identified using a scanning electron microscope (SEM) and thinned with a focused ion-beam (FIB) to produce crystalline lamellae of ideal thickness. Continuous rotation data were collected using an ultra-low exposure rate on a Falcon 4 direct electron detector in electron-counting mode. For the first sample, triclinic lysozyme extending to 0.87 Å resolution, an ideal helical fragment of only three alanine residues provided initial phases. These phases were improved using density modification, allowing the entire atomic structure to be built automatically. A similar approach was successful on a second macromolecular sample, proteinase K, which is much larger and diffracted to 1.5 Å resolution. These results demonstrate that macromolecules can be determined to sub-Ångström resolution by MicroED and that ab initio phasing can be successfully applied to counting data collected on a direct electron detector.
Microcrystal Electron Diffraction of Small Molecules
Raw datasets available for download
- Lysozyme: DOI 10.15785/SBGRID/222
- Catalase: DOI 10.15785/SBGRID/186
- α-synuclein G11A: DOI 10.15785/SBGRID/223
- GSNQNNF: DOI 10.15785/SBGRID/819
- VFAThiaGlu: DOI 10.15785/SBGRID/820
Hardware
The MicroED Stage Controller page contains a full description of the stage rotation controller developed at Janelia Research Campus. This device is not needed on Thermo Fisher systems from 2015 or later.
Software downloads
The software we develop for MicroED is available on the downloads page.
See also
MicroED Imaging Center at UCLA
Selected publications
Clabbers, Max T. B.; Martynowycz, Michael W.; Hattne, Johan; Gonen, Tamir
Hydrogens and hydrogen-bond networks in macromolecular MicroED data
In: J Struct Biol X, vol. 6, pp. 100078, 2022.
Clabbers, Max T. B.; Martynowycz, Michael W.; Hattne, Johan; Nannenga, Brent L.; Gonen, Tamir
Electron-counting MicroED data with the K2 and K3 direct electron detectors
In: J Struct Biol, vol. 214, iss. 4, 2022.
Martynowycz, Michael W; Clabbers, Max T B; Hattne, Johan; Gonen, Tamir
Ab initio phasing macromolecular structures using electron-counted MicroED data
In: Nat Methods, vol. 19, iss. 6, pp. 724–729, 2022, ISSN: 1548-7105.
Martynowycz, Michael W; Clabbers, Max T B; Unge, Johan; Hattne, Johan; Gonen, Tamir
Benchmarking the ideal sample thickness in cryo-EM
In: Proc Natl Acad Sci U S A, vol. 118, no. 49, 2021, ISSN: 1091-6490.
Zhu, Lan; Bu, Guanhong; Jing, Liang; Shi, Dan; Lee, Ming-Yue; Gonen, Tamir; Liu, Wei; Nannenga, Brent L.
Structure Determination from Lipidic Cubic Phase Embedded Microcrystals by MicroED
In: Structure, vol. 28, no. 10, pp. 1149–1159.e4, 2020.
Jones, Christopher G; Martynowycz, Michael W; Hattne, Johan; Fulton, Tyler J; Stoltz, Brian M; Rodriguez, Jose A; Nelson, Hosea M; Gonen, Tamir
The CryoEM Method MicroED as a Powerful Tool for Small Molecule Structure Determination
In: ACS Cent Sci, vol. 4, no. 11, pp. 1587–1592, 2018.
Liu, Shian; Gonen, Tamir
MicroED structure of the NaK ion channel reveals a Na⁺ partition process into the selectivity filter
In: Commun Biol, vol. 1, pp. 38, 2018.
Hattne, Johan; Shi, Dan; Glynn, Calina; Zee, Chih-Te; Gallagher-Jones, Marcus; Martynowycz, Michael W; Rodriguez, Jose A; Gonen, Tamir
Analysis of Global and Site-Specific Radiation Damage in Cryo-EM
In: Structure, vol. 26, no. 5, pp. 759–766, 2018.
de la Cruz, Jason M; Hattne, Johan; Shi, Dan; Seidler, Paul; Rodriguez, Jose; Reyes, Francis E; Sawaya, Michael R; Cascio, Duilio; Weiss, Simon C; Kim, Sun Kyung; Hinck, Cynthia S; Hinck, Andrew P; Calero, Guillermo; Eisenberg, David; Gonen, Tamir
Atomic-resolution structures from fragmented protein crystals with the cryoEM method MicroED
In: Nat. Methods, vol. 14, no. 4, pp. 399–402, 2017.
Hattne, Johan; Shi, Dan; de la Cruz, Jason M; Reyes, Francis E; Gonen, Tamir
Modeling truncated pixel values of faint reflections in MicroED images
In: J Appl Crystallogr, vol. 49, no. Pt 3, pp. 1029–1034, 2016.
Shi, Dan; Nannenga, Brent L; de la Cruz, Jason M; Liu, Jinyang; Sawtelle, Steven; Calero, Guillermo; Reyes, Francis E; Hattne, Johan; Gonen, Tamir
The collection of MicroED data for macromolecular crystallography
In: Nat Protoc, vol. 11, no. 5, pp. 895–904, 2016.
Rodriguez, Jose A; Ivanova, Magdalena I; Sawaya, Michael R; Cascio, Duilio; Reyes, Francis E; Shi, Dan; Sangwan, Smriti; Guenther, Elizabeth L; Johnson, Lisa M; Zhang, Meng; Jiang, Lin; Arbing, Mark A; Nannenga, Brent L; Hattne, Johan; Whitelegge, Julian; Brewster, Aaron S; Messerschmidt, Marc; Boutet, Sébastien; Sauter, Nicholas K; Gonen, Tamir; Eisenberg, David S
Structure of the toxic core of α-synuclein from invisible crystals
In: Nature, vol. 525, no. 7570, pp. 486–490, 2015.
Hattne, Johan; Reyes, Francis E; Nannenga, Brent L; Shi, Dan; de la Cruz, Jason M; Leslie, Andrew G W; Gonen, Tamir
MicroED data collection and processing
In: Acta Crystallogr A Found Adv, vol. 71, no. Pt 4, pp. 353–360, 2015.
Nannenga, Brent L; Shi, Dan; Hattne, Johan; Reyes, Francis E; Gonen, Tamir
Structure of catalase determined by MicroED
In: Elife, vol. 3, pp. e03600, 2014.
Nannenga, Brent L; Shi, Dan; Leslie, Andrew G W; Gonen, Tamir
High-resolution structure determination by continuous-rotation data collection in MicroED
In: Nat. Methods, vol. 11, no. 9, pp. 927–930, 2014.
Iadanza, Matthew G; Gonen, Tamir
A suite of software for processing MicroED data of extremely small protein crystals
In: J Appl Crystallogr, vol. 47, no. Pt 3, pp. 1140–1145, 2014.
Shi, Dan; Nannenga, Brent L; Iadanza, Matthew G; Gonen, Tamir
Three-dimensional electron crystallography of protein microcrystals
In: Elife, vol. 2, pp. e01345, 2013.
Wisedchaisri, Goragot; Gonen, Tamir
Fragment-Based Phase Extension for Three-Dimensional Structure Determination of Membrane Proteins by Electron Crystallography
In: Structure, vol. 19, no. 7, pp. 976–987, 2011.
Relevant reviews and book chapters
Clark, Lisa J.; Bu, Guanhong; Nannenga, Brent L.; Gonen, Tamir
MicroED for the study of protein–ligand interactions and the potential for drug discovery
In: Nat Rev Chem, vol. 5, pp. 853–858, 2021.
Mu, Xuelang; Gillman, Cody; Nguyen, Chi; Gonen, Tamir
An Overview of Microcrystal Electron Diffraction (MicroED)
In: Annu Rev Biochem, vol. 90, pp. 431–450, 2021, ISSN: 1545-4509.
Danelius, E; Gonen, T
Protein and Small Molecule Structure Determination by the Cryo-EM Method MicroED
In: Owens, Raymond J. (Ed.): vol. 2305, pp. 323–342, 2021.
Nannenga, Brent L; Gonen, Tamir
The cryo-EM method microcrystal electron diffraction (MicroED)
In: Nat. Methods, vol. 16, no. 5, pp. 369–379, 2019.
Martynowycz, Michael W; Gonen, Tamir
From electron crystallography of 2D crystals to MicroED of 3D crystals
In: Curr Opin Colloid Interface Sci, vol. 34, pp. 9–16, 2018.
Nannenga, Brent L; Gonen, Tamir
Protein structure determination by MicroED
In: Curr. Opin. Struct. Biol., vol. 27, pp. 24–31, 2014.
Nannenga, Brent L; Iadanza, Matthew G; Vollmar, Breanna S; Gonen, Tamir
Overview of Electron Crystallography of Membrane Proteins: Crystallization and Screening Strategies Using Negative Stain Electron Microscopy
In: Curr Protoc Protein Sci, vol. 72, no. 1, pp. 17.15.1–17.15.11, 2013.
Wisedchaisri, Goragot; Gonen, Tamir
Phasing Electron Diffraction Data by Molecular Replacement: Strategy for Structure Determination and Refinement
In: Electron Crystallography of Soluble and Membrane Proteins, vol. 955, Chapter 14, pp. 243–272, 2012.
Gonen, Tamir
The Collection of High-Resolution Electron Diffraction Data
In: Electron Crystallography of Soluble and Membrane Proteins, vol. 955, Chapter 9, pp. 153–169, 2012.
Stokes, David L; Ubarretxena-Belandia, Iban; Gonen, Tamir; Engel, Andreas
High-Throughput Methods for Electron Crystallography
In: Electron Crystallography of Soluble and Membrane Proteins, vol. 955, Chapter 15, pp. 273–296, 2012.
Wisedchaisri, Goragot; Reichow, Steve L; Gonen, Tamir
Advances in Structural and Functional Analysis of Membrane Proteins by Electron Crystallography
In: Structure, vol. 19, no. 10, pp. 1381–1393, 2011.